MAP Master Amino Acid Pattern® Tablets
![]() Printable Versions: English, Spanish, French
Printable Versions: English, Spanish, French
DESCRIPTION
MAP™ is a dietary protein substitute that provides the MAP Master Amino Acid Pattern® (U.S. Patent No. 5,132,113) a unique pattern of essential amino acids in a highly purified, free, crystalline form. After oral ingestion, MAP™ is rapidly utilized. MAP™ does not require the aid of peptidases and therefore is absorbed within 23 minutes through the first 100 cm of functional small intestine. MAP™ does not provide any fecal residue. MAP™ is amphoteric. MAP™ is supplied in tablets of 1,000 mg for oral administration. Each tablet of MAP™ contains only the active ingredient MAP™. MAP™ contains no inactive ingredients.
COMPOSITION
MAP™, provides the MAP Master Amino Acid Pattern® (U.S. Patent No. 5,132,113) a unique pattern of essential amino acids in a highly purified, free, crystalline form.
CLINICAL STUDIES
The results of comparative, double-blind, triple- and quintuple-crossover Net Nitrogen Utilization (NNU) clinical studies have shown that the subjects, while taking MAP™, as a dietary protein substitute, achieved a body’s 99% NNU. This means that 99% of MAP’s constituent amino acids followed the anabolic pathway, thus acting as precursor of body’s protein synthesis. By comparison, the most nutritious dietary proteins provide an average of only 32% NNU. Hence, MAP™ is more nutritious than dietary proteins. This has been confirmed by the fact that during the studies, each subject body’s nitrogen balance was maintained in equilibrium by taking MAP™ as a sole and total substitute of dietary proteins in a dosage of only 400 mg/kg/day (ideal weight) which provided less than 2 kcal/day ( 1 g MAP™ = 0.04 kcal). The studies results have also shown that 1% of MAP’s constituent amino acids followed the catabolic pathway, thus releasing only 1% of nitrogen catabolites and energy. By comparison dietary proteins release an average of 68% nitrogen catabolites and energy. These facts evidence that MAP™ is safer than dietary proteins and provides the lowest amount of energy in comparison to any dietary protein.
To illustrate: when a dietary protein is digested, it releases its constituent amino acids into the small intestine where they are absorbed. Then, those amino acids can follow either the anabolic pathway or the catabolic pathway (Fig. I).
Figure I. Dietary Protein Metabolism
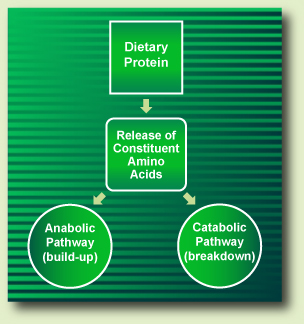
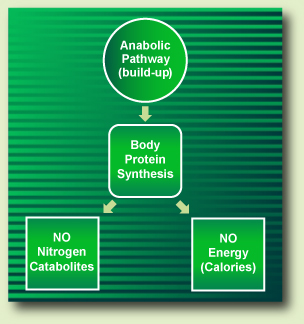
When dietary amino acids follow the anabolic pathway, they act as precursors for the body's protein synthesis (BPS), thus becoming the body's constituent proteins. Throughout the anabolic pathway amino acids do not release any nitrogen catabolites or energy (Fig. II).
Figure II. The Protein Metabolism Anabolic Pathway
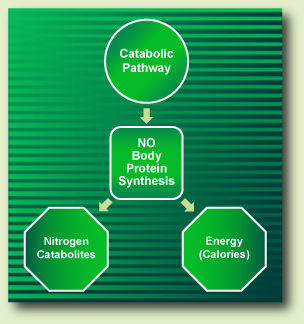
On the other hand, when dietary amino acids follow the catabolic pathway, they act only as a source of energy and not as precursors of body’s proteins synthesis (BPS). Throughout the catabolic pathway, amino acids do release nitrogen catabolites and energy (Fig. III).
Figure III. The Protein Metabolism Catabolic Pathway
INDICATIONS AND USAGE
MAP™ is indicated as a safe and effective substitute for dietary proteins.
| MAP™ vs. Dietary Proteins & Protein Supplements | |||
|---|---|---|---|
| Characteristics | MAP™ | Dietary Proteins |
Protein Supplements |
| Net Nitrogen Utilization (NNU) for Body Protein Synthesis (BPS) | 99% | 32% (average) | 16% (average) |
| Digestion Time | 23 min | 3-6 hours (6-12 times longer) |
3-6 hours (6-12 times longer) |
| BPS/Time NNU/min) | 99% NNU/ 23 min |
24-48 times lower | 48-96 times lower |
| Released Nitrogen Catabolites | 1% | 68% (average) | 84% (average) |
| Energy | 0.04 kcal/g | 4 kcal/g | 4 kcal/g |
| Fecal residue | Absent | Present | Present |
| Contraindications | None | Renal Failure or Hepatic Failure |
Renal Failure or Hepatic Failure |
| Adverse Reactions | None | Food Sensitivities | Food Sensitivities |
| Refrigeration | Not Needed | Needed | N/A |
ADVERSE REACTIONS
No adverse reactions have been reported.
OVERDOSAGE
No adverse reactions have been reported.
DOSAGE AND ADMINISTRATION
MAP™ should be administered with food. MAP™ in a dosage of 400mg/kg/day (ideal weight) has been shown to be adequate as a sole and total substitute of dietary proteins to maintain the body’s nitrogen balance in equilibrium. To calculate the MAP™ dosage necessary to substitute dietary proteins, apply the following:
For instance, to calculate the dosage of MAP™ necessary to substitute 10 g of dietary proteins, proceed as follows:
- MAP™ dosage = (Dietary Proteins x 0.4) g
- MAP™ dosage = (10 x 0.4) g
- MAP™ dosage = 4 g
Therefore, 4 g (4 tablets) of MAP™ provide a body’s protein synthesis (BPS) equivalent to that provided by at least 10 g of the most nutritious dietary protein.
If administering more than 10 tablets per day, increase dosage gradually. (Not more than 10 tablets should be administered within a two hour period. If gastrointestinal disorders are present, pulverize tablets to allow for better absorption.)
SUPPLY INFORMATION
MAP™ is available in bottles of 120 tablets of 1,000 mg, for oral administration.
Additional information
is available for members.
If you would like to become a member,
please
register here and you will
be emailed a
username and password to obtain access to additional information.
If you are an existing member, please login.
For frequently asked questions (FAQs) click here.
Information on this site is provided for informational purposes and is not meant to substitute for the advice provided by your own physician or other medical professional. You should not use the information contained herein for diagnosing or treating a health problem or disease, or prescribing any medication. If you have or suspect that you have a medical problem, promptly contact your health care provider. Information and statements regarding dietary supplements have not been evaluated by the Food and Drug Administration and are not intended to diagnose, treat, cure, or prevent any disease. Customer reviews are provided for informational purposes only. Customer reviews reflect the individual reviewer's results and experiences only and are not verified or endorsed.
All content ©2010 International Nutrition Research Center (INRC). All rights reserved. No part of this website may be reproduced or distributed in any form or by any means, or stored in a database or retrieval system, without the prior written permission of the INRC.
Physicians' Desk Reference® is a registered trademark of Thomson Healthcare Inc.
